From Lab to Bedside: Overcoming the Hurdles to Patient Enrollment in Oncology Research
Despite being crucial for developing new life-saving therapies, many patients are never told about cancer clinical trials. Systemic issues, such as physician time shortages and lack of resources in community clinics, create a communication deficit, hindering patient enrollment and oncology research.

Cancer clinical trials are the primary engine driving the development of new life-saving treatments, yet a significant number of eligible patients are never informed they are an option. While patient participation has increased in recent years, experts report that systemic barriers—including severe time constraints on physicians and a lack of resources, particularly in community settings—mean this critical conversation is often left unspoken, hindering both patient care and the pace of oncology research.
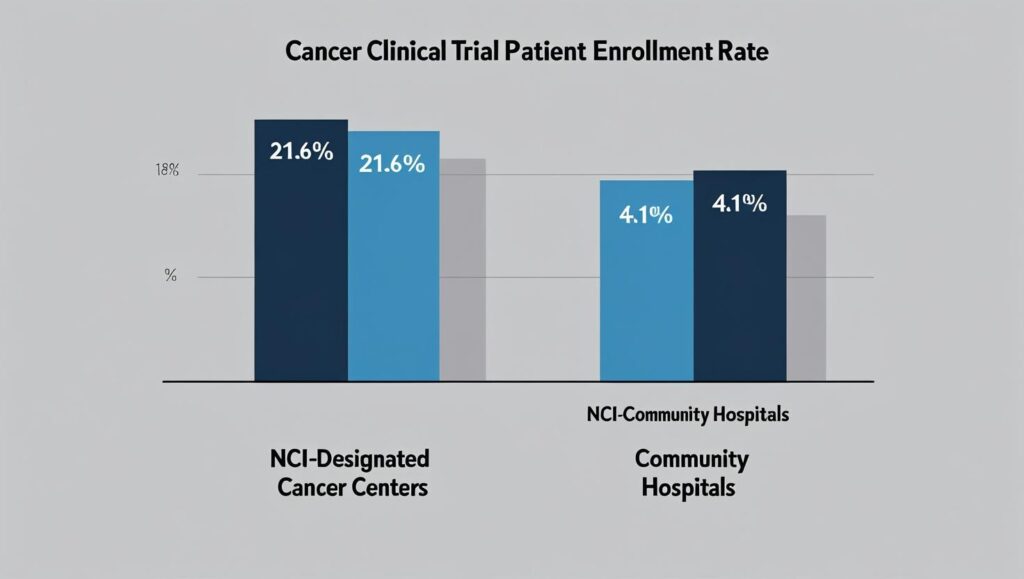
While new data shows improvement, a persistent gap remains. A 2024 study published in the Journal of Clinical Oncology revealed that overall enrollment in cancer treatment trials is approximately 7.1%, higher than historical estimates of 2-3%. However, that rate is dramatically different depending on the setting: 21.6% at National Cancer Institute (NCI)-designated cancer centers versus just 4.1% at community sites, where the majority of U.S. cancer patients receive care.
This disparity highlights a crucial challenge: for many, access to potentially groundbreaking treatment options depends entirely on whether their doctor has the time, knowledge, and resources to even begin the discussion.
Key Insights: Cancer Trial Enrollment
| Key Fact | Detail / Statistic |
| Overall Treatment Trial Enrollment | 7.1% of adult cancer patients participate in treatment-focused trials. |
| The Community Care Gap | Enrollment is over 5 times higher at NCI-designated centers (21.6%) than at community cancer programs (4.1%). |
| Beyond Treatment Trials | When including all types of research (e.g., biorepository, genetic), 1 in 5 (21.9%) patients participates. |
| Primary Physician Barrier | Lack of time and overwhelming administrative workload are cited as major reasons oncologists do not discuss trials. |
Hurdles in the Clinic: Why Doctors Aren’t Talking
For physicians on the front lines of cancer care, the barriers to discussing cancer clinical trials are both numerous and complex. They extend far beyond a simple unwillingness to communicate and are rooted in the structure of modern healthcare.
Time Constraints and Administrative Burden
The most commonly cited obstacle is time. Oncologists, particularly those in busy community practices, face immense pressure to see a high volume of patients, leaving little room for the lengthy, nuanced conversations required to explain a clinical trial.
“We only have two people in our office for all trials,” one physician noted in a 2020 study on the topic published in JCO Oncology Practice. “They are drowning in paperwork, are overwhelmed, [and] are not always available to talk with the patient.”
This administrative overload includes identifying relevant trials, meticulously screening patients against complex eligibility criteria, and managing the extensive documentation required for patient enrollment.
Lack of Awareness and Access
While major academic cancer centers have robust trial portfolios and dedicated research staff, community oncologists may lack access to or awareness of available studies that could benefit their patients.
“Our goals differ from … [those of] tertiary, academic centers,” another community oncologist stated in the same study. “More of our patients come in requiring adjuvant therapy [or] first-line metastatic trials. Those are the trials we like [and need].”
This disconnect means that a patient’s access to a trial often depends on geography and the resources of their treatment facility, creating significant disparities in care.
Misconceptions and Patient Fears
Lingering myths also contribute to the communication gap. Some physicians may hesitate to suggest a trial, fearing patients will feel like a “guinea pig.” Patients, in turn, may harbor misconceptions about receiving a placebo or undergoing unsafe experiments.
However, modern cancer clinical trials are built on a foundation of ethical oversight and patient safety protocols. According to the National Cancer Institute, every trial has a detailed protocol and is monitored by an Institutional Review Board (IRB) to protect participants. In late-stage cancer trials, placebos are rarely used alone if an effective standard treatment is available; instead, a new treatment is often compared directly against the current standard of care.
Bridging the Gap: Solutions and Innovations
Recognizing these barriers, healthcare organizations and technology companies are actively developing solutions to facilitate better communication and wider access to oncology research.
The Role of Technology and Trial Matching
Artificial intelligence (AI) and sophisticated database platforms are emerging as powerful tools to ease the burden on clinicians. These systems can rapidly scan a patient’s electronic health record and match their specific diagnosis and genomic profile against a global database of thousands of available trials.
“AI uses tools like machine learning, natural language processing, and predictions. It can look at big sets of data much faster and more accurately than people can,” explains a report from Simbo.ai on the technology. Companies like ConcertAI and Tempus have developed platforms that can reduce patient screening time significantly, freeing up clinicians to focus on the patient conversation.
Decentralizing Trials to Expand Access
The COVID-19 pandemic accelerated the adoption of decentralized clinical trials (DCTs), a model that allows many trial-related activities to occur at or near a patient’s home. By leveraging telemedicine, local clinics, and mobile health technologies, DCTs reduce the travel and financial burden on patients who do not live near major research centers.
“A major benefit of DCTs is the ability to reach underserved and marginalized communities in ways that traditional trials cannot,” according to an analysis by ObvioHealth. This model makes it easier for community oncologists to refer and co-manage patients, democratizing access to cutting-edge treatment options.
The American Society of Clinical Oncology (ASCO) provides clear guidelines for patient-clinician communication, emphasizing that clinicians should make patients aware of all treatment options, including clinical trials, so they can make informed decisions aligned with their personal goals.
As oncology research becomes more personalized and complex, the need for this dialogue will only grow. The path forward, experts agree, requires a multi-pronged approach: empowering physicians with better tools, redesigning trial logistics to be more patient-centric, and fostering a healthcare culture where discussing clinical trials is not the exception, but the standard.